| Conference | Abstract Open | Abstract Deadline | Start Date | End Date | Location |
|---|---|---|---|---|---|
| Association of Professors of Gynecology & Obstetrics (APGO) Faculty Development Seminar | January 10, 2026 | January 13, 2026 | Amelia Island, Florida | ||
| Health Professions Education Conference | February 1, 2025 | February 1, 2025 | Honolulu, Hawaii | ||
| OB/GYN Clinical and Surgical Updates | February 5, 2026 | February 8, 2026 | Kamuela, Hawaii | ||
| International Hawaii Cardiovascular Symposium (IHCVS) | February 6, 2025 | February 8, 2025 | Honolulu, Hawaii | ||
| Society for Maternal-Fetal Medicine (SMFM) | May 1, 2025 | August 4, 2025 | February 8, 2026 | February 13, 2026 | Las Vegas, Nevada |
| The Council on Resident Education in Obstetrics and Gynecology (CREOG) and the Association of Professors of Gynecology and Obstetrics (APOG) Annual Meeting | February 18, 2026 | February 21, 2026 | Seattle, Washington | ||
| ACGME Annual Education Conference | Late Summer | Early Fall | February 19, 2026 | February 21, 2026 | San Diego, California |
| Society for Reproductive Investigation (SRI) | October 10, 2025 | March 24, 2026 | March 28, 2026 | San Juan, Puerto Rico | |
| JABSOM Biomedical Sciences & Health Symposium | April 4, 2025 | April 5, 2025 | Honolulu, Hawaii | ||
| American Society of Addiction Medicine (ASAM) | April 23, 2026 | April 26, 2026 | San Diego, California | ||
| American College of Obstetricians and Gynecologists (ACOG) | May 16, 2025 | May 18, 2025 | Minneapolis, Minnesota | ||
| The Association of Women's Health, Obstetric and Neonatal Nurses (AWHONN) | June 21, 2025 | June 25, 2025 | Orlando, Florida | ||
| Society for the Study of Reproduction (SSR) | May 15, 2025 | July 29, 2025 | August 1, 2025 | Washington, DC | |
| Hawaii Health Workforce Summit | September 6, 2025 | September 6, 2025 | Honolulu, Hawaii | ||
| The Society for Academic Specialists in General Obstetrics and Gynecology (SASGOG) | September 11, 2025 | September 12, 2025 | Denver, Colorado | ||
| Society of Family Planning | April 9, 2025 | October 25, 2025 | October 27, 2025 | Pittsburgh, Pennslyvania | |
| American Society of Reproductive Medicine (ASRM) | April 30, 2025 | October 25, 2025 | October 29, 2025 | San Antonio, Texas | |
| Annual Fall Conference on Obstetrics | October 29, 2025 | November 1, 2025 | Big Island, Hawaii | ||
| Annual Fall Conference on Issues in Women's Health | November 12, 2025 | November 16, 2025 | Maui, Hawaii |
Effective July 1, 2016, all new research protocol applications (excludes Cooperative studies) must be submitted through the eProtocol system.
Determine Whether or Not Your Project is Human Subjects Research
To help you determine whether your project is considered human subjects research, download Worksheet 301- Is My Project “Human Subjects Research?”.
Choose your Application Form
To help you determine whether your research qualifies for EXEMPT, EXPEDITED, or FULL-BOARD review, use the Review Category Flowchart.
Below are links to the various application forms you will need:
Complete the application form according to the instructions provided within the form
Submit completed application to the Human Studies Program
Exempt and expedited applications are reviewed in the order that they are received. See the Full-board submission deadlines.
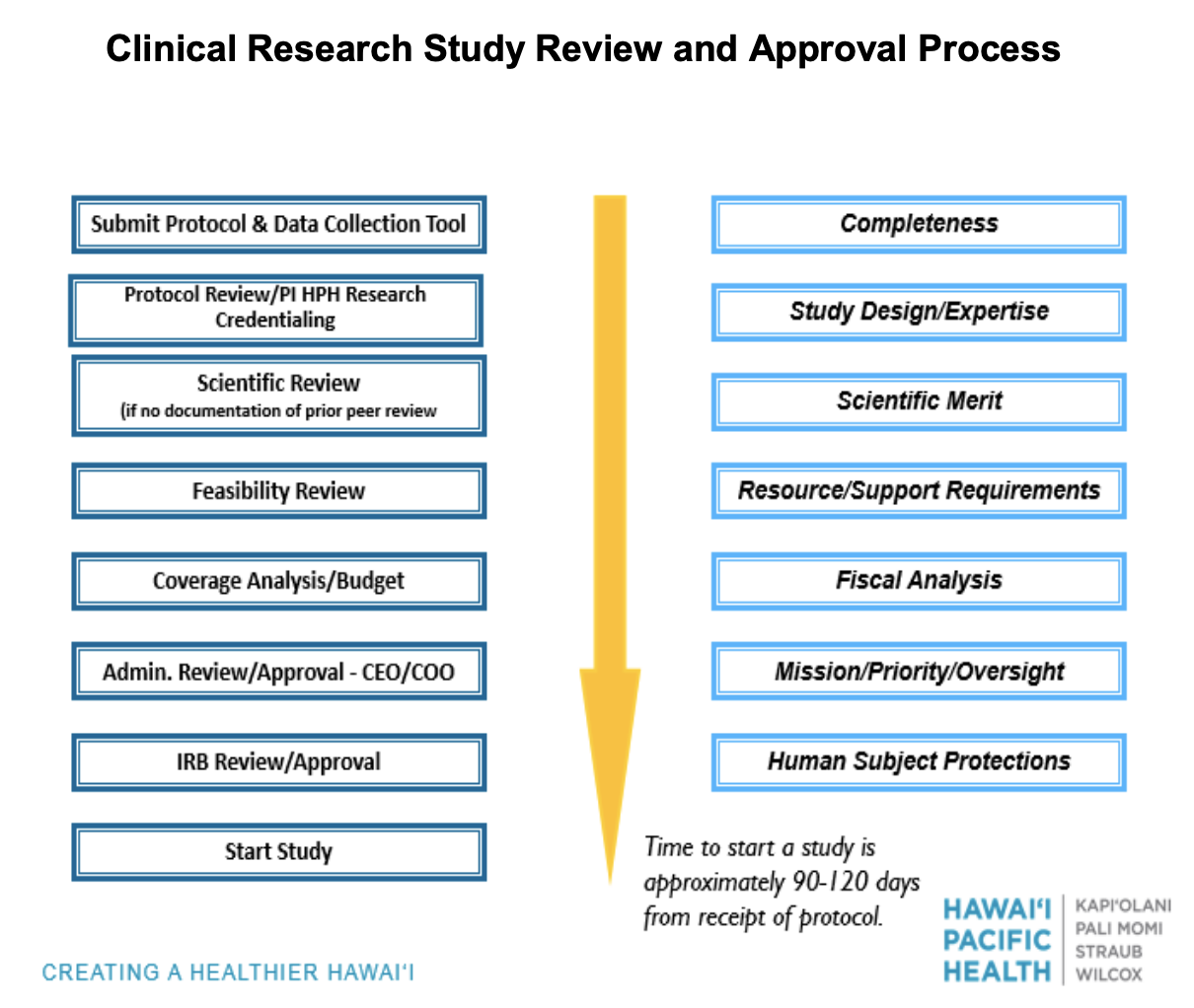
Submit a complete or draft protocol with data collection sheet to HPHRI at research@hawaiipacifichealth.org for a preliminary review of your project. After the preliminary review is complete, a member of the HPHRI team will contact you to complete the required forms for your specific project.
All research conducted at Hawaii Pacific Health must have scientific review by the Scientific Review Committee. HPHRI will submit your project to the Scientific Review Committee on your behalf and contact you with the results of the review.
Should your project require full IRB review, the HPHRI team will assist you with the submission to the Western IRB.
Hawaii Pacific Health uses the Western Institutional Review board as its IRB.
To assure that all clinical researchers understand their responsibility to protect the welfare of their research subjects, Hawaii Pacific Health requires that researchers be “certified” in human subjects protection before conducting research.